Natural or Unnatural?
We're told that life on Earth formed first with no oxygen
(02), but that this early life then produced the
oxygen required for
life as we know it today. Does the Miller-Urey experiment offer a
"natural" explanation for the beginnings of life? Take a look and decide for yourself:
Was the process natural?
We first have to ask if the apparatus used for the
experiment seems to emulate a natural system.
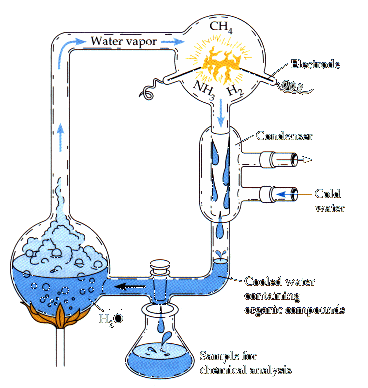
- Water was boiled to create water vapor, simulating heated gases from the
Earth's core. By contrast, volcanoes today emit water vapor and
carbon dioxide (CO2)
and nitrogen.
- It was passed through a chamber with gases that provide the building
blocks for amino acids, but which are extremely rare in the atmosphere
today. The gases were rich in hydrogen,
even though our present atmosphere is 80% nitrogen. No oxygen was allowed because
it is a very reactive gas which quickly combines with other gases and
which would break down any organic compounds created.
- The heated gases were sparked with an electrode, simulating lightning.
- The molecules formed in the reaction were immediately cooled and
then trapped in a collection chamber, simulating the oceans. Otherwise, the heat would
break down the molecules back into gases.
What do you think?
Natural or not so natural?
We also have to ask:
Was the atmosphere natural?
OR
The composition of the Earth's present atmosphere is as follows:
| N2 (nitrogen) |
78.084% |
| O2 (oxygen) |
20.946% |
| H2O (water) |
0-5% |
| Ar (argon) |
0.934% |
| CO2 (carbon dioxide) |
0.034% |
| Ne (neon) |
0.0018% |
| He (helium) |
0.0005% |
| Kr (krypton) |
0.0003% |
| CH4 (methane) |
0.00017% |
| H2 (hydrogen) |
0.00006% |
| N2O (nitrous oxide) |
0.00003% |
| Xe (xenon) |
0.000009% |
| O3 (ozone) |
0.000004% |
| Particles (dust and soot) |
0.000001% |
| CFC (chlorofluorocarbons) |
0.00000001% |
Note that of the gases used in the experiment, methane (CH4
) represents only 0.00017% of the Earth's present atmosphere, hydrogen (H2)
represents only 0.00006% and anhydrous ammonia (NH3) is virtually
non-existent. Ammonia, in fact, is highly caustic and when produced
industrially poses a significant risk to humans and livestock and can damage the
environment.
What do you think?
Natural or not so natural?
Did the elements required for the experiment come not from the early
atmosphere, but from the Earth itself? Water (H20)covers 71% of the Earth's
surface and chemical composition (by mass) of the rest of the Earth is:
| Iron |
34.6% |
|
Oxygen
|
29.5%
|
| Silicon |
15.2% |
| Magnesium |
12.7% |
| Nickel |
2.4% |
| Sulfur |
1.9% |
| Titanium |
0.05% |
Although oxygen would have kept the Miller-Urey reaction from succeeding,
it now represents 20.9% of the atmosphere and 29.5% of the solid matter in
the Earth's composition.
In the end, nobody knows for sure what the Earth's early atmosphere was
like. We just know that in order to assume that life began on its own,
we also have to assume that it must have been ENTIRELY different than
the Earth we observe today.
What do you think?
Natural or not so natural?
Note: In July 1999, Scientific American
presented a new theory that life on Earth was seeded by comets and reported
that "New evidence has drawn the components of Miller's
atmosphere into question."
Still, it was the basis of scientific belief
for almost two generations.
|

